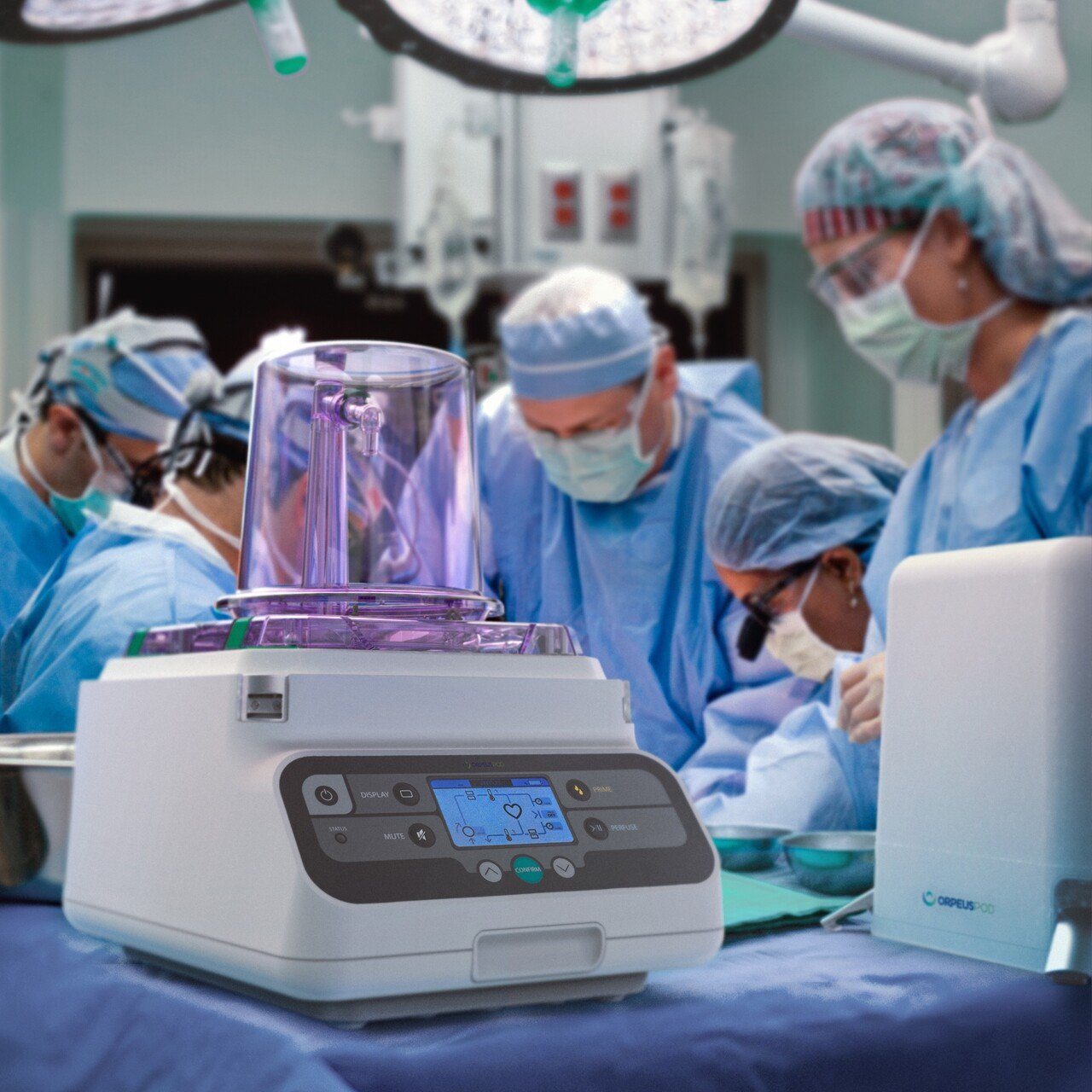
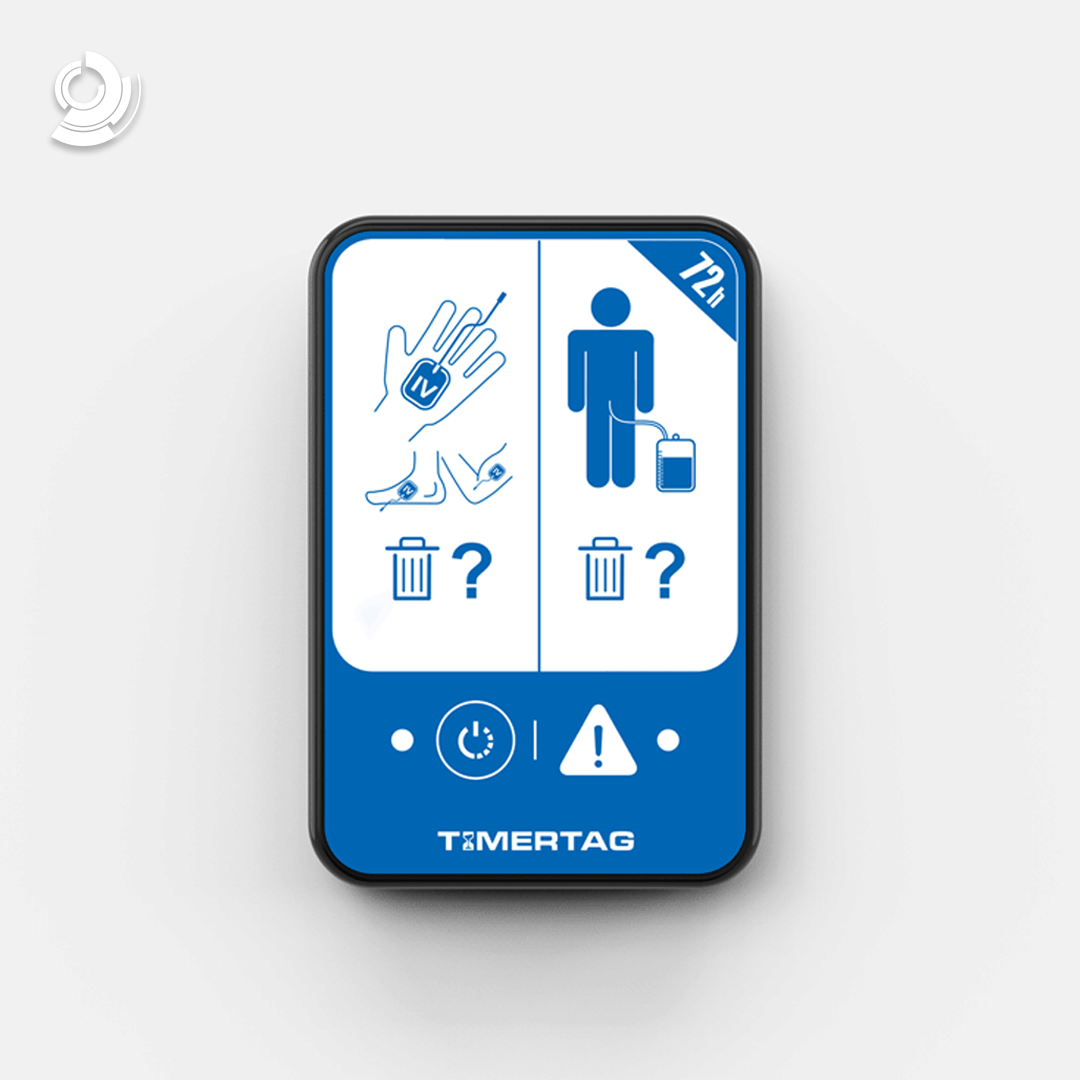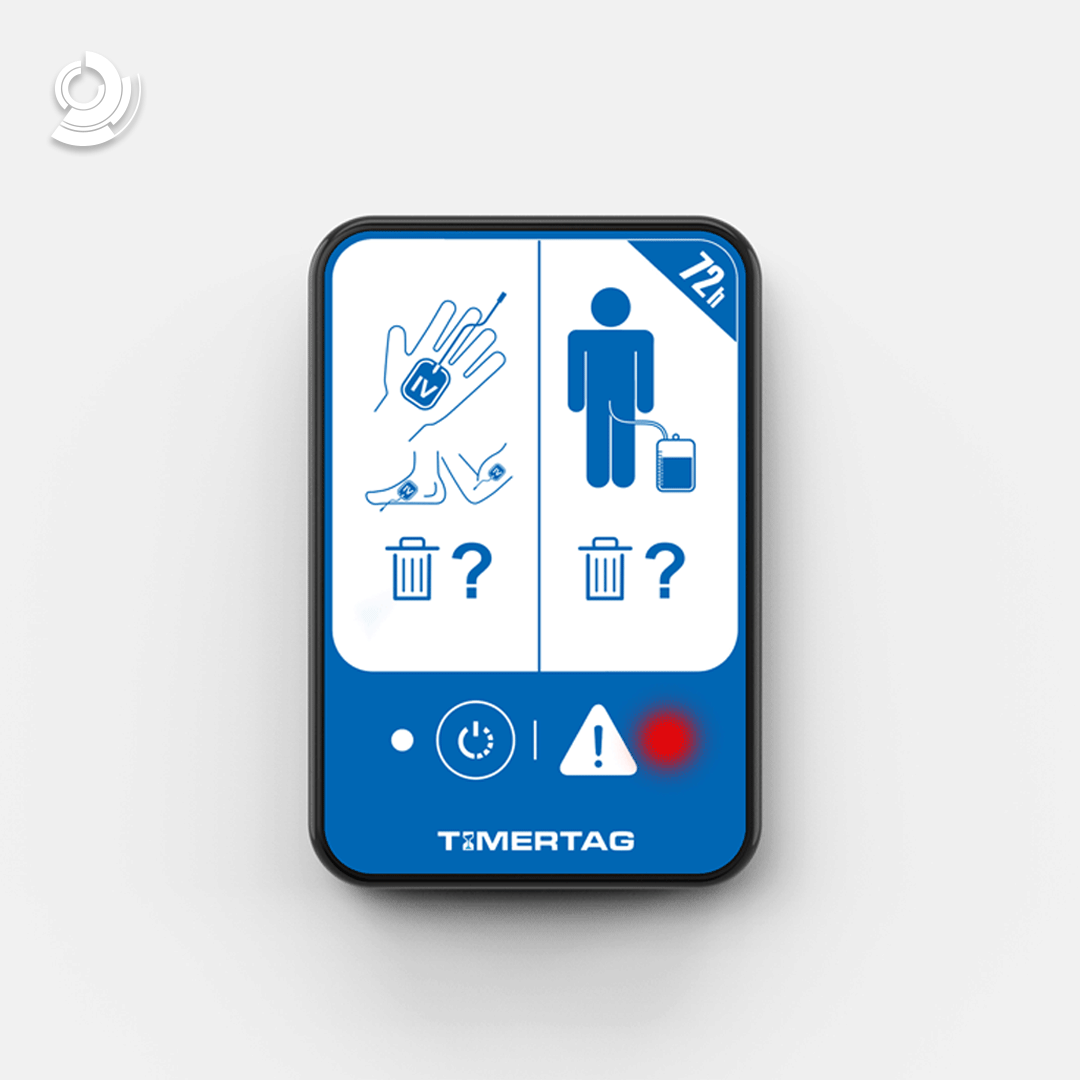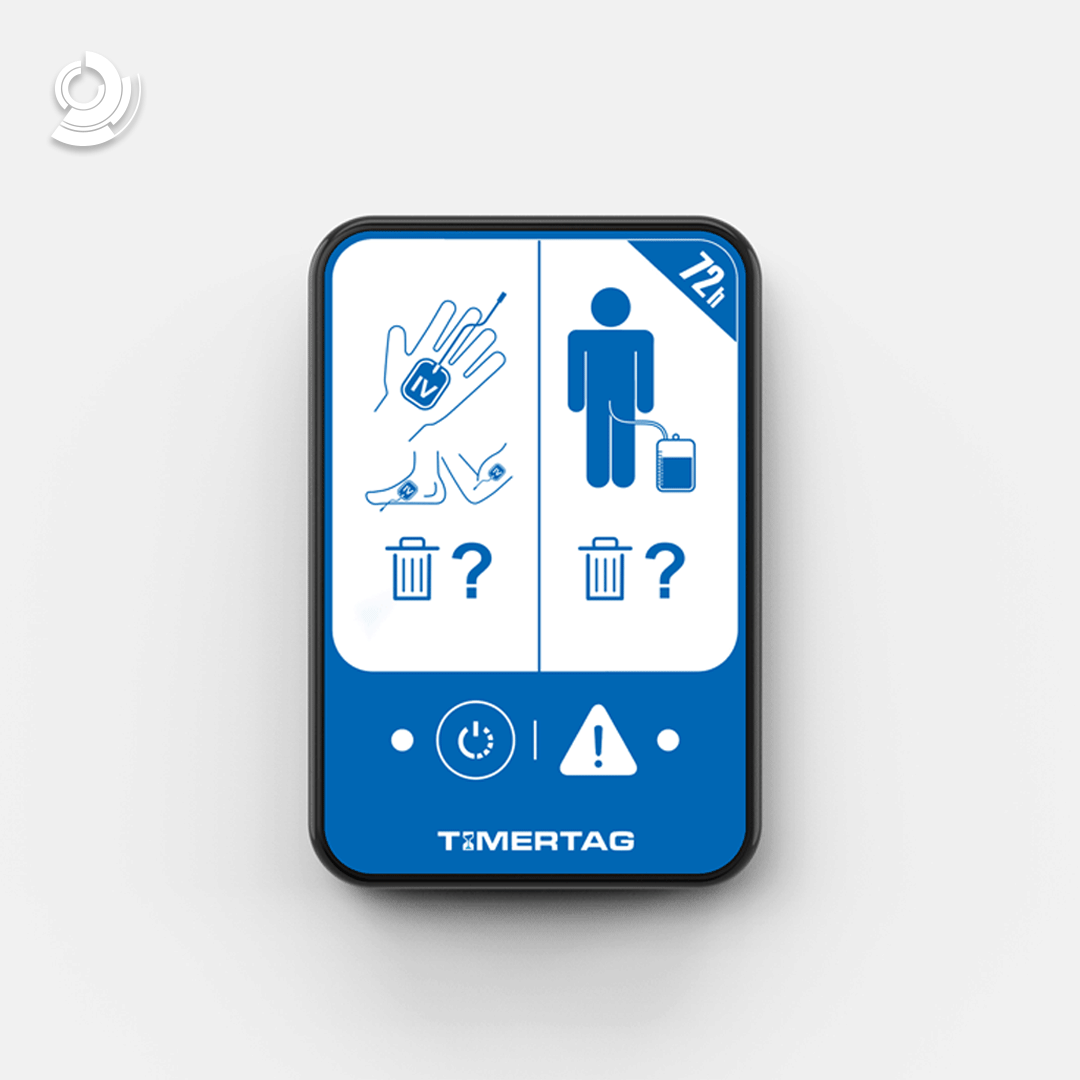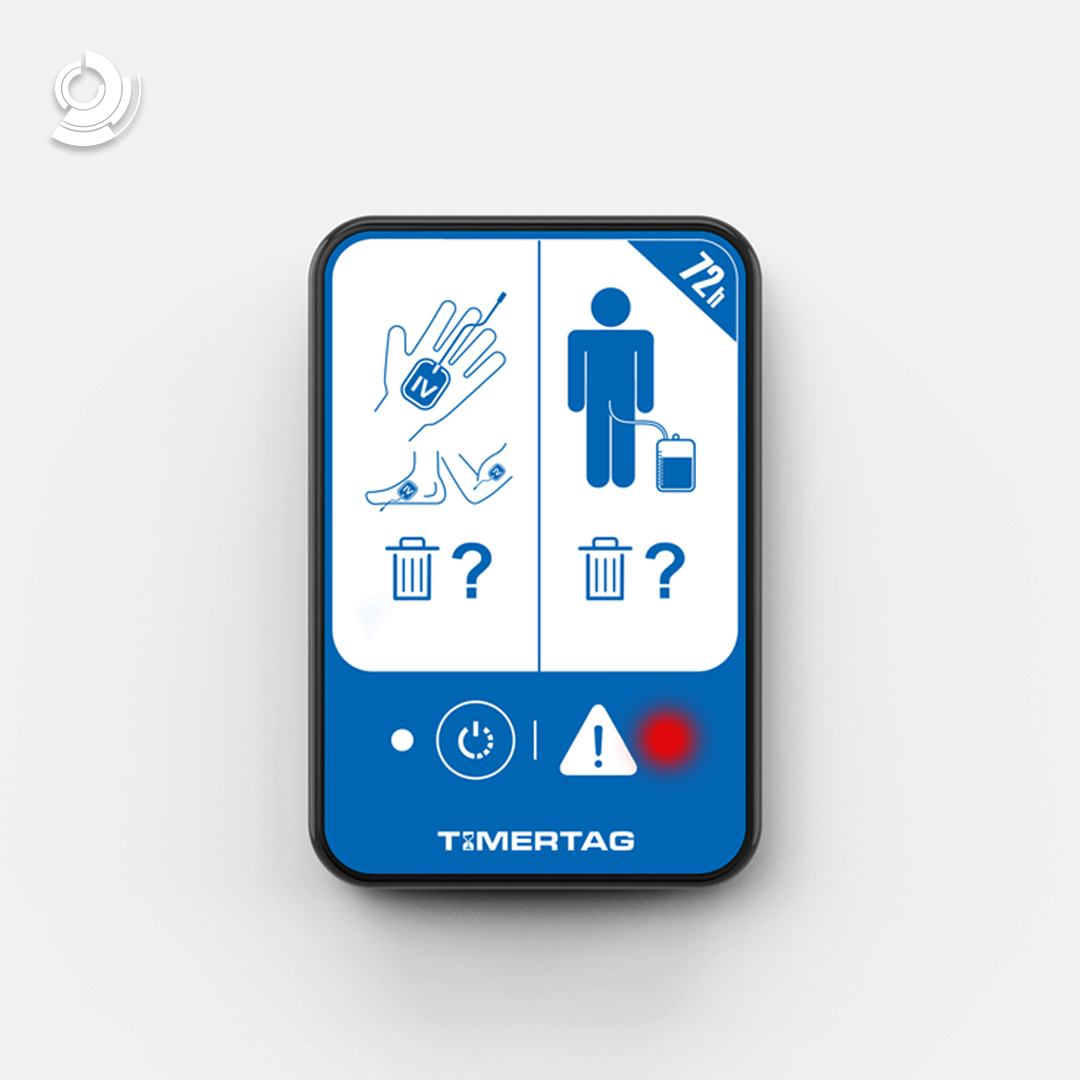
About Design + Industry
Our services include design, mechanical engineering, electronics, software and firmware development, management into production, and regulatory compliance. We understand the unique challenges that arise in the development of scientific and medical devices and equipment, and we have the proven experience to help your organisation navigate these challenges successfully.
Our Quality Management System is ISO 13485 certified, which ensures that our standards for safety, usability, operability, and compliance are met, and that your products meet the requirements of the medical industry and users. With our commitment to quality and excellence, we can help you bring new and innovative products to market and improve the lives of people around the world.
Our clients
We've worked with large multi-national clients and next-generation Australian Startups to develop best-in-class product solutions: Siemens Healthcare, GAMA Healthcare, Alcolizer, Trajan Scientific and Medical, AdvanCell, DJO Chattanooga, Universal Biosensors, Biorithm, Navbit, Signostics, Micro-X and CliniCloud.
Inventia Life Sceience Rastrum 3D Bioprinter: Good Design Award of the Year Winner 2019.
This exceptional bioprinter allows the user to print models of living cells in 3D. An award-winning device, this technology has a wide range of applications from medical research to pharmaceuticals to customised patient care. The bioprinter offers a wealth of new possibilities for the treatment of cancer and other diseases.
AdvanCell Isotopes – World-first alpha isotope generator which addresses the greatest unmet need in targeted alpha therapy, the reliable and scalable supply of isotope:
The AdvanCell ²¹²Pb Generator is a proprietary manufacturing platform capable of delivering a scalable solution to fully automated, GMP compliant manufacture of targeted alpha therapies. The manufacture of clinical doses of a high-value isotope Alpha 212® (Lead-212) for use in targeted radionuclides therapy is a game-changer for prostate and several other cancer treatments.
Client Testimonial: “The genesis of the Company arose from an insight that Julian Kelly, AdvanCell’s Chief Nuclear Scientist, had about a novel method to produce an isotope with properties well suited for cancer treatment. With the support of our shareholders and the NSW Health Medical Devices Fund, D+I and AdvanCell managed to design and build this generator and produce our first isotope from it – in under 12-months. Drug design and pre-clinical studies followed shortly after, and we aim to begin a first in human clinical trial before year end.” - Andrew Adamovich (Chief Executive Officer, AdvanCell)
Lenexa Medical – The Internet of Medical Things (IoMT) – A Connected-Care Solution helping to prevent and monitor Pressure Injuries in Healthcare Environments.
The LenexaCARE solution is a world-first fabric-based sensor technology paired with AI software that monitors patient position and pressures in real-time to enable personalised pressure injury (or ‘bed sore’) prevention. The system provides patient-specific non-subjective data to guide clinicians and carers to deliver a higher quality of care, working to minimise patient suffering and to reduce the multi-billion-dollar healthcare burden associated with preventable pressure injuries.
BioRithm – Maternal + Fetal Wellbeing Wearable Monitor: Remote Patient Monitoring.
FeMom is designed to capture essential maternal and fetal bio-signals to determine the health of the mother and baby in a clinical and home setting. The Femom monitor leverages on Non-Invasive Fetal Electrocardiogram (NIFECG) and Electrohysterography (EHG) monitoring. Femom helps clinicians to identify early signs of risks before they become emergencies, working to ensure a safer and smoother journey for mother and baby.
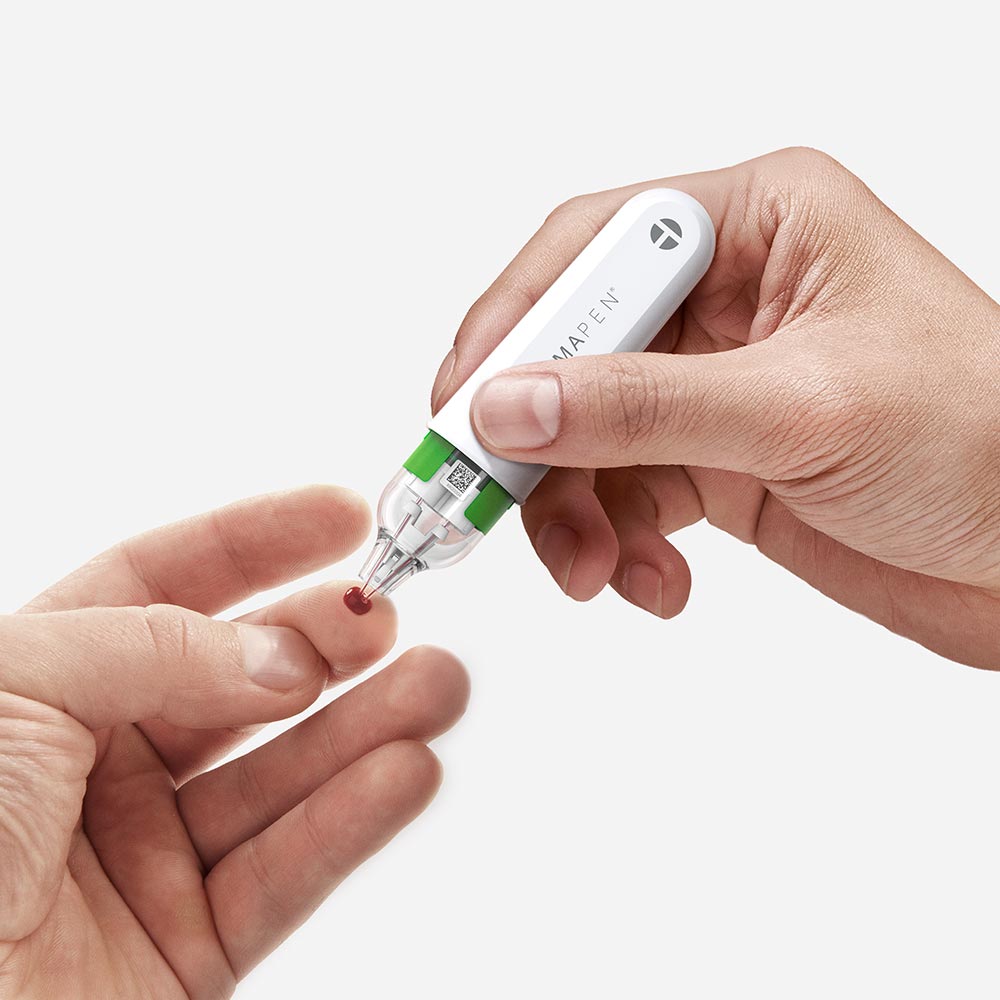
hemaPEN® - Point-of-care Diagnostics Sample Collection.
The ultra-convenient hemaPEN® allows precise micro-samples of blood to be collected by almost anyone, anywhere at any time. This exceptional tool was developed by researchers based at the University of Tasmania through proof-of-concept, commercialised for mass-production and compliance by D+I, and has recently been included on the Australian Register of Therapeutic Goods.
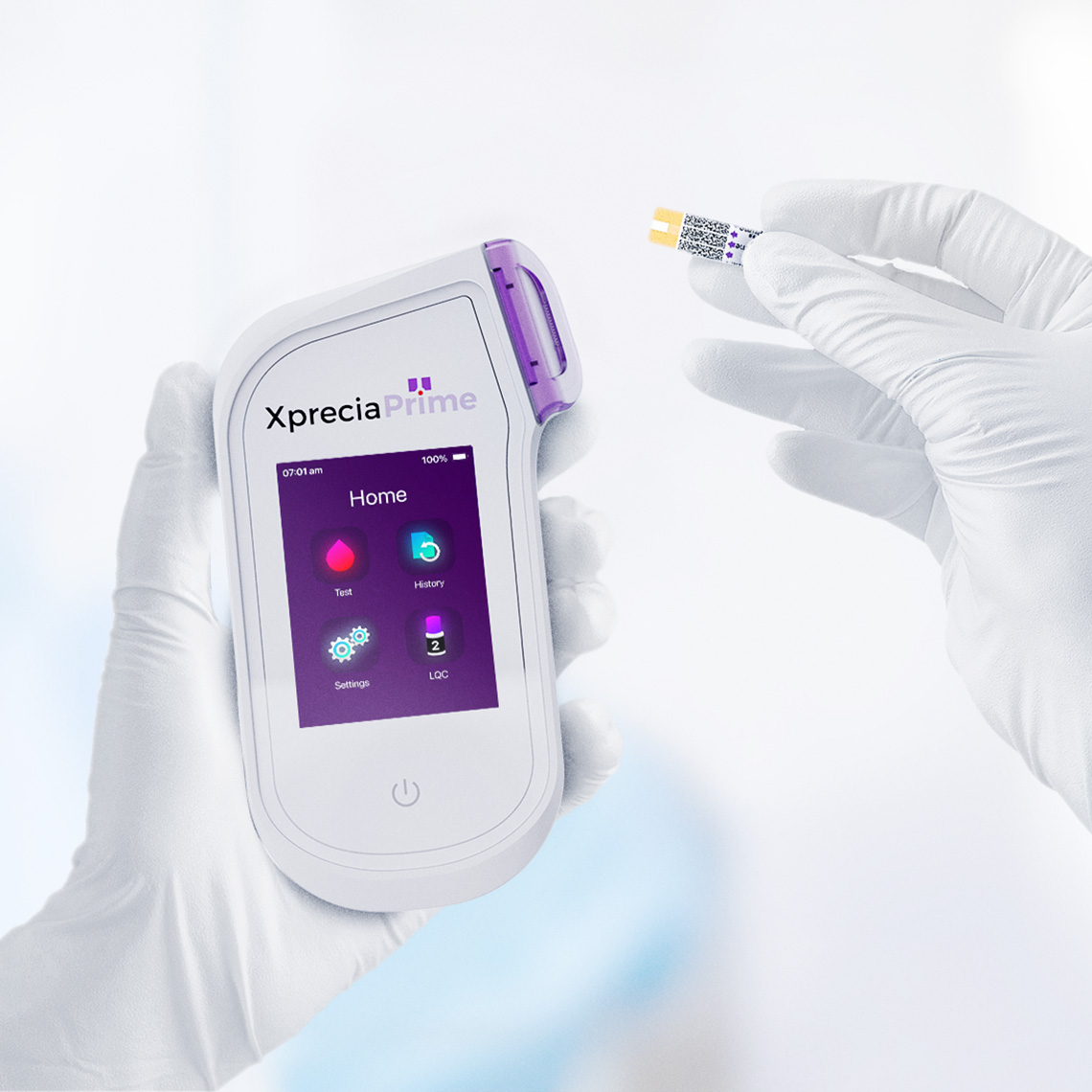
Siemens Xprecia Stride - Point-of-care sample analysis and diagnostics.
This award-winning device is the result of a collaboration between D+I, Siemens Healthineers and Universal Biosensors. Xprecia Stride™ allows for reliable analysis of blood coagulation at point-of-care, transforming the testing experience for both patients and medical professionals.
Local + International Industry Recognition:
D+I has been recognised with over 270+ industry awards for design and engineering excellence.
Our accolades in medical product development include the prestigious: Good Design Award of the Year - Advancell Isotope Generator (2022) , Red Dot Best of the Best (2022) - Rediroom Instant Patient Isolation Room, and Good Design Best in Class Award: Engineering – AbilityMade Photogrammetry Scanner for the creation of custom orthoses (2020).
Starting a project?
Are you looking to be at the forefront of the medical technology revolution by investing in the compliant design and engineering of a next-generation medical device or product that will have a transformative impact on the healthcare sector? If so, we invite you to reach out to us today to learn more about how our team of experienced and highly skilled designers, engineers, and regulatory compliance experts can help you bring your ideas to life.
Contact us online or visit one of our offices in Melbourne, Sydney, or Newcastle — Let's work together to bring your product to life, and elevate the quality of care for patients who need it most.
Phone:
+61 2 9555 1166
Email:
info@design-industry.com.au